the pictorial version of periodic table song:
in english: http://www.youtube.com/watch?v=sm1uxjGm_N0
in Japanese: http://www.youtube.com/watch?v=ljsUVDOcYB0
Website where all the pictures in the videos come from:
http://periodictable.com/Elements/061/index.html
into the Rafflesian Chemist's mind
Monday, February 7, 2011
Sec 2 Chem: Acids and Bases- The Chemistry of Reactions
In essence
Acids react with:
· Bases to form a salt and water
- Hydrochloric Acid + Sodium Hydroxide à Sodium Chloride + Water
- HCl + NaOH à NaCl + H2O
· Carbonates to form a salt, carbon dioxide and water
- Hydrochloric Acids + Potassium Carbonate à Carbon Dioxide + Water + Potassium Chloride
- HCl + K2CO3 à CO2 + H2O + 2KCl
· Metals to form a salt and Hydrogen gas
- Sulfuric acid + Magnesium à Hydrogen Gas + Magnesium Sulfate
- H2SO4 + Mg à H2 + MgSO4
· Sulfites to form a salt, Sulfur Dioxide and Water
- Sulfuric Acid + Magnesium Sulfite à Sulfur Dioxide + Water + Magnesium Sulfate
- H2SO4 + MgSO4 à SO2 + H2O + MgSO4
Reaction of Bases: Note that Ammonia (NH3) and oxides of metals are bases too.
Bases react with:
· Acids to form a salt and water
- No need example, eh? Its same as above…
· Ammonium salts (Salts with the NH4 component) to form another salt, Ammonia gas and Water
- Sodium Hydroxide + Ammonium Chloride à Ammonia + Water + Sodium Chloride
- NaOH + NH4Cl à NH3 + H2O + NaCl
· Certain salt solutions (Non- Group I or Ammonium) to form an insoluble hydroxide* and another salt
- Potassium Hydroxide + Copper (II) Sulfate à Potassium Sulfate + Copper (II) Hydroxide
- 2KOH + CuSO4 à K2SO4 + Cu(OH)2
You may wish to know what so special about the hydroxide, or about Grp I and ammonium salts. Refer to the post “Solubility rules”, in future….
*Types of Oxides
Oxides of Non-metals are either acidic or neutral Oxides of metals are usually basic and oxides of lead, zinc and aluminum are amphoteric. This means that they can act as BOTH acids and bases in reactions, depending on what it is reacted with.
Acidic Oxides | Basic Oxides | Amphoteric Oxides | Neutral Oxides |
Carbon Dioxide | Cupper (II) Oxide | Beryllium | Carbon Monoxide |
Sulfur Dioxide | Sodium Oxide | Tin | Nitrogen Monoxide |
Nitrogen Dioxide | Iron (II) Oxide | Zinc | |
Iron (III) Oxide | Lead | ||
Magnesium Oxide | Aluminum |
Reactions of Amphoteric Oxides:
As a base, reaction with acids:
Zinc Oxide + Hydrochloric Acid à Water + Zinc Chloride
ZnO+ 2HCl à H2O + ZnCl2
As an acid, with bases:
Zinc Oxide + Sodium Hydroxide + Water (I Dunno what’s the water doing here)à Sodium Zincate
ZnO + 2NaOH + H2O à Na2 Zn(OH)4
Sec 2 Chem: Structure and Properties
As requested, heres the notes on structure and properties. (Quite self-explanatory, but there are a couple common misconceptions)
Suppose this is all we need to know for now....the rest should also be self-explanatory (Overly used phrase in the post...)
Ionic Bonding
One thing you need to know about ionic compounds before understanding structure and properties:
Ionic compounds have structures in the form of a giant lattice. That is, the + and – ions involved extend forever and ever……
From the above model, although it seems that there is a definite number of ions in an ionic compound, say NaCl, the structure actually extends infinitively. Therefore, the chemical formula is NOT the number of ions involved, but the ratio of ions involved. In this case, Na = Cl hence the formula NaCl.
Also, in ionic bonding, a + charged ion (Cation) is attracted to ANY – charged ion, or anion. The anion a cation is attracted to NEED NOT be the recipient of the electron. (Remember? Electron donors bond with electron receivers) This means, the electrons donated by each metal are “re-distributed” randomly to random non-metal atoms. For example, you may donate a hamper to a charitable organisation. The organisation collates all the collections and distribute them randomly to random recipients……(Yes, something like SGB..lol)
So, ionic compounds do not consist of molecules, unlike covalent substances. Covalent substances have chemical formula that IS the number of atoms present.
Ionic compounds,
Have ions as basic particles | as, duh?!---there are electrostatic forces bet ions, ionic bonds (An interruption by the totally irrelevant singlish version): Of course ions lah?!?! Ionic compounds mah...wad u expect...covalent or harry potter magical glue issit??? (No offence intended...for entertainment only) |
Have high melting and boiling points | As there are strong electrostatic forces between each oppositely charged ion. Much heat is required to break these bonds. Here’s a higher-order thinking question: Can you suggest why the melting point of Sodium Chloride is lower than Magnesium Oxide? Think in terms of bonding and Periodic Group number! |
Cannot conduct electricity at solids state | As all the charged particles, in this case, ions. Are involved in bonding. There are no mobile charged particles to carry electric current. |
But can conduct electricity at liquid and aqueous (Dissolved) state | As through melting and dissolving, the + and – ions are being separated and they are mobile. Note, in melting, HEAT seperates the + and – ions. In dissolving, the water molecules act as agents to split up the ions. (The oxygen end of the molecule, being more electron-loving, attracts the Na+ while the H ends attract the Cl-) |
Covalent Bonding
There is a need to differentiate between a covalent compound, and a simple covalent substance:
Covalent compounds refer to ONE single molecule that the atoms in it are involved in covalent bonding. Whereas simple covalent compounds refer to A NUMBER of similar molecules. (E.g. compare a single water molecule with a jug of water)
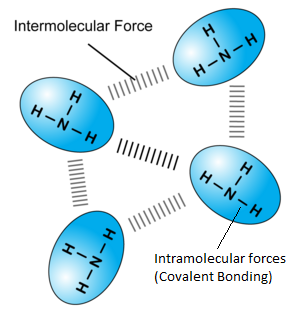
While in covalent compounds, the only force/bonds involved is covalent bonds between atoms. However, in simple covalent substances, there are both intermolecular and intra-molecular forces. Intra molecular forces refer to forces WITHIN a molecule, and in this case, the covalent bond. Inter-molecular forces refer to forces that act between each molecule, that determines distance between each molecule and hence the state of the substance. One intermolecular force is van de Waals forces, generated due to the fast and constantly moving of electrons in each molecule. Take this ammonia substance for example:
Now we have established the commonly confused points, let get into the content :D
Simple Covalent Substances:
Have molecules as their basic particle | As each molecule contributes to the overall simple covalent substance. They are held together by van de Waals forces. |
Have low boiling and melting points | The intermolecular forces are weak (As they are temporary dipole interactions, oh just ignore this…..Sec 3 content) and are easily broken by the heat energy supplied during state change. The molecules can separate easily and hence change state. (Remember Particulate nature of matter, in Sec 1?) HOWEVER< bear in mind that when a substance change state, the MOLECULES and NOT INDIVIDUAL ATOMS separate. When you boil water you get steam, which is still H2O. You don’t separate the H and O to get hydrogen and oxygen gas!!! The heat required to change state (Overcome the intermolecular forces) are NOT strong enough to break the intramolecular forces, or covalent bonds. In other words, covalent bonds ARE strong!!! |
Do not conduct electricity at all 3 states | ah, this is self-explanatory. Refer to ionic compound properties |
Suppose this is all we need to know for now....the rest should also be self-explanatory (Overly used phrase in the post...)
Sunday, February 6, 2011
Sec 2 Chem: Acids and Bases (Introduction)
Commons acids in the Science lab include:
Hydrochloric Acid | HCl |
Sulfuric Acid | H2SO4 |
Nitric Acids | HNO3 |
Note: Amino acids are not acids! Only acidic amino acids are!
A characteristic of acids is that they dissociate into H+ ions in water (Aqueous): For example:
HCl à H+ + Cl-
H2SO4 à 2H+ + SO42-
Note that dissociation ereactions are reversible, hence the àsign should be replaced by the following symbol, which cannot be types out on blogspot :
It is the weird double-headed arrow in the centre...just ingore the A and B and S and T...dunno how to crop....
Strong acids are those that dissociate fully in water. Weak acids are those that partially dissociate. For example, ethanoic acid, CH3COOH, dissociates in water to form CH3COO- and H+ ions. However, only 4% of the molecules dissociates.
The basicity of an acid is the number of H+ ions they dissociate in water. (Note: Ethanoic Acids has a basicity of 1 istead of 4, as the H3 remains together when in water)
The scientist Svante Arrhenius proposed that acids dissociates in water to form H+ ions. Hence, a compound that exhibit this character is called an Arrhenius acid.
As for bases, here are some commons ones:
Sodium Hydroxide | NaOH |
Calcium Hydroxide | Ca(OH)2 |
Arrhenius’ definition of a base is a compound that dissociates to form OH- ions in water.
However, compounds such as Ammonia, NH3 and Cooper Oxide, CuO, are regarded as bases too, despite not having an OH- ion. Hence Arrhenius’ model went into some difficulties (See “Changing models of Acids and Bases”)
Saturday, February 5, 2011
Sec 2 Chem: Ionic and Covalent Bonding
Ionic Bonding
All atoms have a neutral net charge due to same number of protons and electrons present in the atom itself. However, the atoms’ charges can change due to electron transfer. When an atom gains one electron, is becomes negatively charged. Conversely, when it loses an electron, it becomes positively charged. Losing/ gaining one electron causes the atom to have a charge of 1+ or 1-. What about losing or gaining 2 or more? This is self-explanatory :P
* A positively charged ion is called a cation. A negatively charged on is called an anion.
Ionic bonds are formed between charged atoms where one loses electron and another gains. Hence, ionic compounds have a neutral charge. The ions are held together due to the electrostatic forces between the opposite charged particles.
For example, Sodium, with electronic configuration (EC) 2.8.1, loses 1 electron to obtain a noble gas EC (Stable octet structure, remember?); Chlorine, 2.7, receives 1 electron and its EC becomes one of a noble gas. As both are positively and negatively charges (1+ and 1-), there charges offset each other and they combine to form the compound sodium chloride, NaCl.
Na à Na+
Cl à Cl-
Na+ + Cl- à NaCl
Most commonly, metals lose electrons while non-metals gain electrons (Remember the periodic table and electronegativity?) But what determines how many electrons are transferred? For metals, it is the Group number: Group I metals lose 1 electron to obtain EC of noble gas, Group II loses 2 electrons, Group III loses 3. On the other hand, non-metals, the electrons they gain ic commonly 8 minus their group number. For instance, Chlorine gains 8-7=1 electrons while oxygen gains 8-6=2 electrons. So, for an interaction between Grp II and VI elements:
Ca à Ca2+
O à O2-
Ca2+ + O2- à CaO
Notice that for both compounds discussed, the ratio of cations and anion is 1:1. This is because, as mentioned, ionic compounds have neutral overall charges: Hence 2+ offsets 2-, 3+ offsets 3- (But this rarely happens as much energy is needed to transfer 3 elcetrons)
So, what about ions with different charges? Let’s take Na+ and O2-.
Well, if you had paid attention during maths lesson, you would have came across the term Lowest Common Multiple. Ionic bonding utilizes this concept too! To offset the 2- charge on oxygen, 2 Na+ ions are required, hence yielding the compound Na2O.
2 Na+ (This means 2 sodium ions) + O2- à Na2O
Here’s another problem: Aluminum Oxide.
From the name, we know the compound is made of 2 ions: Aluminum and oxygen. Aluminum, being in group 3, has an ion with a net charge of 3+. Oxygen has a net charge of 2-.
To offset the charges, we use the common LCM method: 3+ x 2 offset 2- x 3
Hence, the formula for aluminum oxide is Al2O3.
#A list of common cations and anions:
Common Cations | |||
Name of ion Formula Other names | |||
Al3+ | |||
Ca2+ | |||
Copper(II) | Cu2+ | cupric | |
H+ | |||
Iron(II) | Fe2+ | ferrous | |
Iron(III) | Fe3+ | ferric | |
Mg2+ | |||
Mercury(II) | Hg2+ | mercuric | |
K+ | kalic | ||
Ag+ | |||
Na+ | natric | ||
Simple Anions | ||
Cl− | ||
F− | ||
O2− | ||
*Polyatomic ions: These ions are made of more than one atom covalently bonded to one another. Yes, these ions are covalent compounds, but the ionic compound they form with opposite charge ions is ionic, and there is an overall net charge on the entire covalent compound. Take Copper (II) Sulphate:
Copper has a charge of 2+, hence a Sulphate ion would have a charge of 2-. Sulphate has a formula of SO42-. This means one Sulphur atom is bonded to 4 oxygen atoms, with an OVERALL charge of 2-. To simplify this, lets break down the sulphate ion into 2 parts: SO3 and O.
SO3 is sulphur trioxide, a covalent compound with a neutral net charge. When bonded to another extra O2- ion, it becomes SO4 with a charge of 2-. Hence the formula SO42-.
Let’s take a look at positive ions: The ammonium ion. The ammonium ion has the formula NH4-. When we break it down, it becomes NH3 (Ammonia gas) and H+ ion. When ammonia gas reacts with a proton (H+ ion), it yields the polyatomic ions ammonium. It can from here form ionic compound with other anions.
#A list of polyatomic ions:
Acetate (ethanoate) | CH3COO− or C2H3O2- |
C6H5COO− or C7H5O2- | |
Bicarbonate (hydrogen carbonate) | HCO3- |
CO32- | |
CN− | |
OH− | |
NO3- | |
PO43- | |
SO42− | |
NH4+ | |
Hydronium or Oxonium | H3O+ |
Hg22+ | |
C7H7+ | |
# The more you look at these and get exposed to these terms, the easier it gets to remember the charges! :D
Covalent Bonding
Now here’s covalent bonding: In covalent bonding, electrons are shared instead of donated and received. Covalent bonds can involve a single bond (One pair of electrons shared), double triple…… But what determines how many electrons are shared? Again, it’s the group number the element is in. As covalent bonds usually only occurs among non-metals, the number of bonds an atom forms would be 8- its group number. For example, oxygen can form 8-6=2 bonds while carbon can form 8-4=4 bonds. Just like ionic bonds, covalent bonds allow atoms to achieve a noble gas electron configuration.
For example, in chlorine gas, Cl2, each atom need to gain 1 more electron (8-7=1). Hence, a double bond is formed between them:
What about more complex compounds: Methane (CH4) is made up of 1 carbon and 4 hydrogen atoms. The carbon atom can form 4 covalent bonds while each hydrogen atom can only form one, hence the structure of methane is:
Oxygen, O2, consist of 2 oxygen atoms which form 2 covalent bonds. However, sine there are only this 2 atoms, they form a double bond instead of single bond, so each atom gains 2 electrons to obtain a noble gas configuration:
Another way of representing compounds, other than the dot-and-cross diagram and chemical formula, is the structural formula. For example, Chlorine would be Cl-Cl, where the - denotes a single bond between each chlorine atom. Oxygen is O=O where = is denoted as a single bond.
Subscribe to:
Comments (Atom)