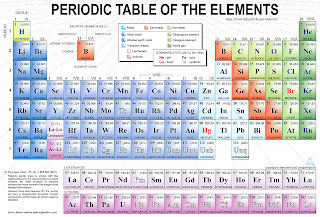
The periodic table is devised by the Russian chemist Dimitri Mendeleev.
Well, the table basically arranges elements in order of increasing atomic numbers (Though it was atomic mass at first). Each horizontal row is a period and each vertical column is a group.
Mendeleev realized that there are many trends in the periodic table and used to predict the properties of Germanium, correctly. :D
Names are given for a reason:
Alkaline Metals: Form alkaline compounds when mixed with water
Alkaline Earth Metals: Found on Earth, occurring naturally
*Transition Metals: Changes their oxidation state (Variable). (Look it up!)
Halogens: (Halo) means salt-forming. They form salts when reacted with substances like metals.
Noble Gases: The “guai”, civilized and noble gases that to not react easily.
Pattern 1: Number of electron shells and valence electrons
The period where an element is situated corresponds with the number of electron shells it contains. For example, Sodium, found on Period 3, has 3 electron shells. The group number corresponds to the number of valence electrons (Outer shell electrons) the atom has. Note that groups are in roman numerals. For instance, Sodium from Group I has 1 valence electron. The noble gases (Group 0) has either 2 or 8 valence electrons (Due to Helium).
Pattern 2: Metallic Characteristic (How metal-like an element is)
As we move across a period on the table, an element changes from a metal to a semimetal/ metalloid and into a non-metal. Metals tend to give up electrons and non-metals tend to accept them
*Pattern 3: Electron transfer (Reduction and Oxidation)
In essence: Oxidation is the LOSS (OIL) of electrons while reduction is the gain (RIG).
A substance that gives up an electron to another is oxidized (OIL). As the other substance is reduced due to the gain, the giver is called a reducing agent.
A substance that accepts an electron from another is reduced (RIG). As the other substance is oxidized to the loss, the acceptor is called an oxidizing agent.
Well, since metals tend to lose electrons (In Pattern 2), we can conclude that as we move across the period, the ability to act as a reducing agent decreases, while the tendency to be reduced increases.
And vice versa:
Well, since metals tend to lose electrons (In Pattern 2), we can conclude that as we move across the period, the ability to act as a oxidizing agent increases, while the tendency to be oxidized decreases.
Pattern 4: Atomic Size (Or Atomic Radius)
 Across a period: Recall that the nucleus is positively charged due to presence of protons and that electrons that orbit around the nucleus (Which determines atomic radius) are negatively charged. Both attract one another as they are opposite net charges. Hence, as we move across the period, the proton number increases, inducing a larger electrostatic force on the electrons. The electrons are drawn nearer towards the nucleus, and hence reduced the atomic radius when we move from left to right.
Across a period: Recall that the nucleus is positively charged due to presence of protons and that electrons that orbit around the nucleus (Which determines atomic radius) are negatively charged. Both attract one another as they are opposite net charges. Hence, as we move across the period, the proton number increases, inducing a larger electrostatic force on the electrons. The electrons are drawn nearer towards the nucleus, and hence reduced the atomic radius when we move from left to right.Down a group: Well, the period number says it all. As the number of electron shells increase, the radius increases too. J
*Pattern 5: First Ionization Energy
1st Ionization Energy (1st IE) is the amount of energy needed to remove 1 unit of electrons from 1 unit of an element. It increases from left to right across a period due to the increasing proton number, hence the electrostatic force acting on the electrons and more energy is required to remove an electron.
*Pattern 6: Electronegativity
This is how much an atom loves electrons. Can you explain why it increases from left to right, also?
Specific Groups in the Periodic Table and trends:
Group/ Property | Group I: Alkaline Metals | *Transition Metals | Group VII: Halogens | Group : Noble Gases |
Colour | Silvery and shiny metals. | Varies, but form different coloured solutions when ions are dissolved: Cu3+ : Orange-brown Cu2+: Dirty Green Cu2+: Blue | Darkens down the group: From Flourine (Yellow) to Chlorine (Y-Green) to Bromine (Reddish Brown) to Iodine (Dark Purple) | Colourless Gases |
Melting Point/ Boiling Point | Decreases down the group (Due to increasing number of electron shells: Less electrostatic forces in between each atom in the metal) Some theories suggest that Caesium may be liquid while Francium may be gas!! | Varies, but transition metals seem to have higher boiling and melting points that Group I and II metals. | Increases down the group. (As the increase in number of electron shells would have a larger surface area of interaction between each diatomic molecule is larger, where more intermolecular forces hold the molecules together.) | Increases down a group, same reason as Halogens, just that Noble Gases are monoatomic. |
Reactivity | Increases down the group. Grp I metals react by losing an electron. With more electron shells shielding the valence electron, less energy is needed to remove the electron, hence increase in reactivity. | Varies; refer to metal reactivity scale: (Not all metals are recorded) | Decreases down the group. This is opposite of Alkaline Metals. (Halogens react by gaining electrons, hence with less electron shells, the tendency to accept an electron from a reaction increases) | Not reactive. This is due to the elements’ stable octet structure: NOT that the valence shell is filled. (Krypton and Xenon’s valence shell in incomplete) However, Xenon is known to form compounds like XeF4. |
Electrical Conductivity | Yes (Metallic atoms are arranged in rows above one another. Each metal atom gives up (an) electron(s) and the atoms are held together by metallic bonding—this is the 3rd type of bonding, between the + charged atom and the mobile electrons. The electrons are small and could move freely among the metal atoms; the atoms are said to be in a sea of electrons. Hence, the electrons are mobile charged particles to carry electric current) | Yes | No. All electrons are involved in covalent bonding of molecules and there are no mobile charged particles. | No |
Density | Lower than transition metals. | Very dense metals compared to Groups I and II. | Density increases down the group, as substances changes from gas to liquid to solid (At room conditions). | Density increases down the group. |
Typical type of bonding | Ionic (with a non-metal) Metallic (Within same element) | Ionic (with a non-metal) Metallic (Within same element) | Covalent (Intramolecular) Van de Waals forces (Intermolecular) | Van de Waals forces only as noble gases do not form molecules. |
Others | More reactive than transition metals. | Diatomic Molecules | Monoatomic elements |

No comments:
Post a Comment