Ionic Bonding
One thing you need to know about ionic compounds before understanding structure and properties:
Ionic compounds have structures in the form of a giant lattice. That is, the + and – ions involved extend forever and ever……
From the above model, although it seems that there is a definite number of ions in an ionic compound, say NaCl, the structure actually extends infinitively. Therefore, the chemical formula is NOT the number of ions involved, but the ratio of ions involved. In this case, Na = Cl hence the formula NaCl.
Also, in ionic bonding, a + charged ion (Cation) is attracted to ANY – charged ion, or anion. The anion a cation is attracted to NEED NOT be the recipient of the electron. (Remember? Electron donors bond with electron receivers) This means, the electrons donated by each metal are “re-distributed” randomly to random non-metal atoms. For example, you may donate a hamper to a charitable organisation. The organisation collates all the collections and distribute them randomly to random recipients……(Yes, something like SGB..lol)
So, ionic compounds do not consist of molecules, unlike covalent substances. Covalent substances have chemical formula that IS the number of atoms present.
Ionic compounds,
Have ions as basic particles | as, duh?!---there are electrostatic forces bet ions, ionic bonds (An interruption by the totally irrelevant singlish version): Of course ions lah?!?! Ionic compounds mah...wad u expect...covalent or harry potter magical glue issit??? (No offence intended...for entertainment only) |
Have high melting and boiling points | As there are strong electrostatic forces between each oppositely charged ion. Much heat is required to break these bonds. Here’s a higher-order thinking question: Can you suggest why the melting point of Sodium Chloride is lower than Magnesium Oxide? Think in terms of bonding and Periodic Group number! |
Cannot conduct electricity at solids state | As all the charged particles, in this case, ions. Are involved in bonding. There are no mobile charged particles to carry electric current. |
But can conduct electricity at liquid and aqueous (Dissolved) state | As through melting and dissolving, the + and – ions are being separated and they are mobile. Note, in melting, HEAT seperates the + and – ions. In dissolving, the water molecules act as agents to split up the ions. (The oxygen end of the molecule, being more electron-loving, attracts the Na+ while the H ends attract the Cl-) |
Covalent Bonding
There is a need to differentiate between a covalent compound, and a simple covalent substance:
Covalent compounds refer to ONE single molecule that the atoms in it are involved in covalent bonding. Whereas simple covalent compounds refer to A NUMBER of similar molecules. (E.g. compare a single water molecule with a jug of water)
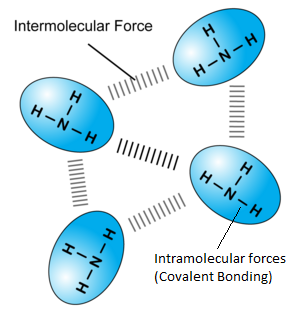
While in covalent compounds, the only force/bonds involved is covalent bonds between atoms. However, in simple covalent substances, there are both intermolecular and intra-molecular forces. Intra molecular forces refer to forces WITHIN a molecule, and in this case, the covalent bond. Inter-molecular forces refer to forces that act between each molecule, that determines distance between each molecule and hence the state of the substance. One intermolecular force is van de Waals forces, generated due to the fast and constantly moving of electrons in each molecule. Take this ammonia substance for example:
Now we have established the commonly confused points, let get into the content :D
Simple Covalent Substances:
Have molecules as their basic particle | As each molecule contributes to the overall simple covalent substance. They are held together by van de Waals forces. |
Have low boiling and melting points | The intermolecular forces are weak (As they are temporary dipole interactions, oh just ignore this…..Sec 3 content) and are easily broken by the heat energy supplied during state change. The molecules can separate easily and hence change state. (Remember Particulate nature of matter, in Sec 1?) HOWEVER< bear in mind that when a substance change state, the MOLECULES and NOT INDIVIDUAL ATOMS separate. When you boil water you get steam, which is still H2O. You don’t separate the H and O to get hydrogen and oxygen gas!!! The heat required to change state (Overcome the intermolecular forces) are NOT strong enough to break the intramolecular forces, or covalent bonds. In other words, covalent bonds ARE strong!!! |
Do not conduct electricity at all 3 states | ah, this is self-explanatory. Refer to ionic compound properties |
Suppose this is all we need to know for now....the rest should also be self-explanatory (Overly used phrase in the post...)

No comments:
Post a Comment