Each individual atom is mainly made up for 3 components: neutrons, electrons and protons. The protons and neutrons make up the nucleus of the atom, while electrons orbit around the nucleus at very high speeds. If a stadium is the size of an atom, the nucleus is as big as a pea placed in the centre.
All atoms are neutral: this means that they have a net charge of 0. Study the comparison below:
Particle | Electron | Proton | Neutron |
Relative Charge | -1 | +1 | 0 |
In any atom, the number of electrons and protons is the same. Hence the overall net charge is zero.
Each atom has a relative mass. It is contributed by the sum of protons and neutrons (Called the nucleon number). Each has a relative mass of 1. The relative mass of an electron (1/1863) are so small that they are not taken in account.
Particle | Electron | Proton | Neutron |
Relative Mass | 1/1836 (Or 0) | 1 | 1 |
A
X
Z
X is the symbol of the element. A is the nucleon number of relative atomic mass, mass number, etc. Z is the proton number of atomic number. (Note the difference between Atomic number and Atomic Mass number)
X is the symbol of the element. A is the nucleon number of relative atomic mass, mass number, etc. Z is the proton number of atomic number. (Note the difference between Atomic number and Atomic Mass number)
Isotopes are atoms of the same element (Same proton number) but different neutron, hence nucleon, number. They have similar chemical properties (Due to same number of electrons) but different physical properties due to different atomic masses.
The 3 main isotopes of hydrogen are: Hydrogen-1, Hydrogen-2 and Hydrogen-3:
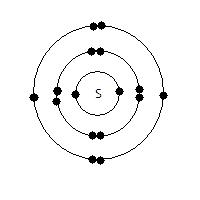 Electrons are arranged in regular orbits (called electron shells) around the nucleus. The nearer the shell is to the nucleus, the lower the energy level. For the first 20 elements, the first shell occupies 2 electron maximum, and subsequent shells (Up to the forth) occupies 8 each. After the 20th element, the number of electrons a shell can hold is based on the formula: 2n2, where n is the nth shell away from the nucleus. The electronic configuration of an atom is shown below: As the name suggests, the figure includes the element and the arrangement of electrons, using a dot and cross diagram. (Since its only 1 element, only 1 symbol, normally crosses, is used.)
Electrons are arranged in regular orbits (called electron shells) around the nucleus. The nearer the shell is to the nucleus, the lower the energy level. For the first 20 elements, the first shell occupies 2 electron maximum, and subsequent shells (Up to the forth) occupies 8 each. After the 20th element, the number of electrons a shell can hold is based on the formula: 2n2, where n is the nth shell away from the nucleus. The electronic configuration of an atom is shown below: As the name suggests, the figure includes the element and the arrangement of electrons, using a dot and cross diagram. (Since its only 1 element, only 1 symbol, normally crosses, is used.)S: The symbol for the element: Sulphur. It has 16 electrons, hence 16 protons too, of which 6 are valence electrons: i.e. the number of electrons on the outermost shell. These are those involved in bonding. Can you explain why isotopes are therefore similar in chemical properties?
The electronic configuration of this atom can be written as: 2.8.6 Do you know why?

No comments:
Post a Comment